Biomass Derived Fluorine-Free Ionic Liquids-Based Electrolytes Enabling Sustainable Batteries
Supercapacitors also called as electric double-layer capacitors (EDLCs) have attracted much attention in the last few decades due to their excellent electrochemical characteristics such as high gravimetric capacitance, high power density, a large number of life cycles and a quick charge-discharge operating processes.
Ionic liquids based electrolytes have been extensively studied in supercapacitors in order to improve the safety and performance of such devices, owning to their excellent physicochemical properties such as low vapor pressure, high ionic conductivity, high thermal and chemical stability and a wide electrochemical window.
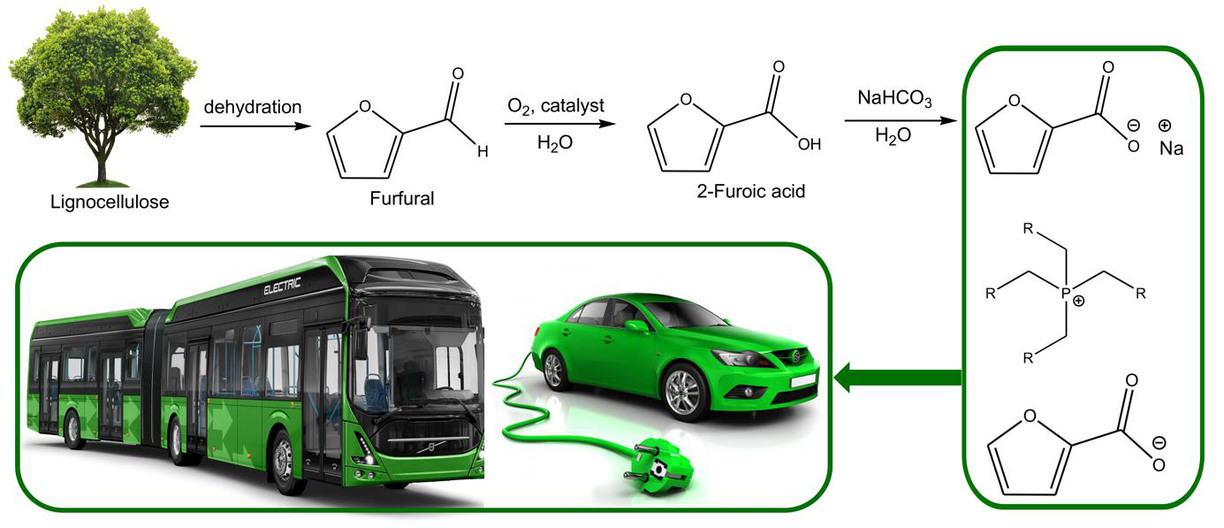
This project is focused on the development of new halogen-free ionic liquids based electrolytes for supercapacitors operating over a wide range of temperature and voltage. The electrolytes are synthesized from pre-cursors, which are produced from biomass on large scale. The ionic liquids s are characterized using multinuclear liquid Nuclear magnetic resonance spectroscopy (NMR), and solid-sate NMR, Pulse-Field Gradient (PFG) NMR, FTIR, MS, Raman spectroscopic techniques. Important physicochemical properties of newly synthesized electrolytes such as thermal behaviour (thermal stability, glass-transition, solid-solid transition (for plastic crystals) and decomposition temperatures) are measured using Thermogravimetric Analysis/Differential Scanning Calorimetry (TG/DSC), while density and viscosity are determined using densitometer and microviscometer, water content are determined using Karl Fisher titration. The electrochemical properties of the ionic liquids are characterized using potentiostat/galvanostat coupled with an Autolab Microcell HC for temperature-controlled experiments.
The project is financed by the Kempe Foundation.
Contact
Faiz Ullah Shah
- Professor
- 0920-491291
- faiz.ullah@ltu.se
- Faiz Ullah Shah
Updated:
