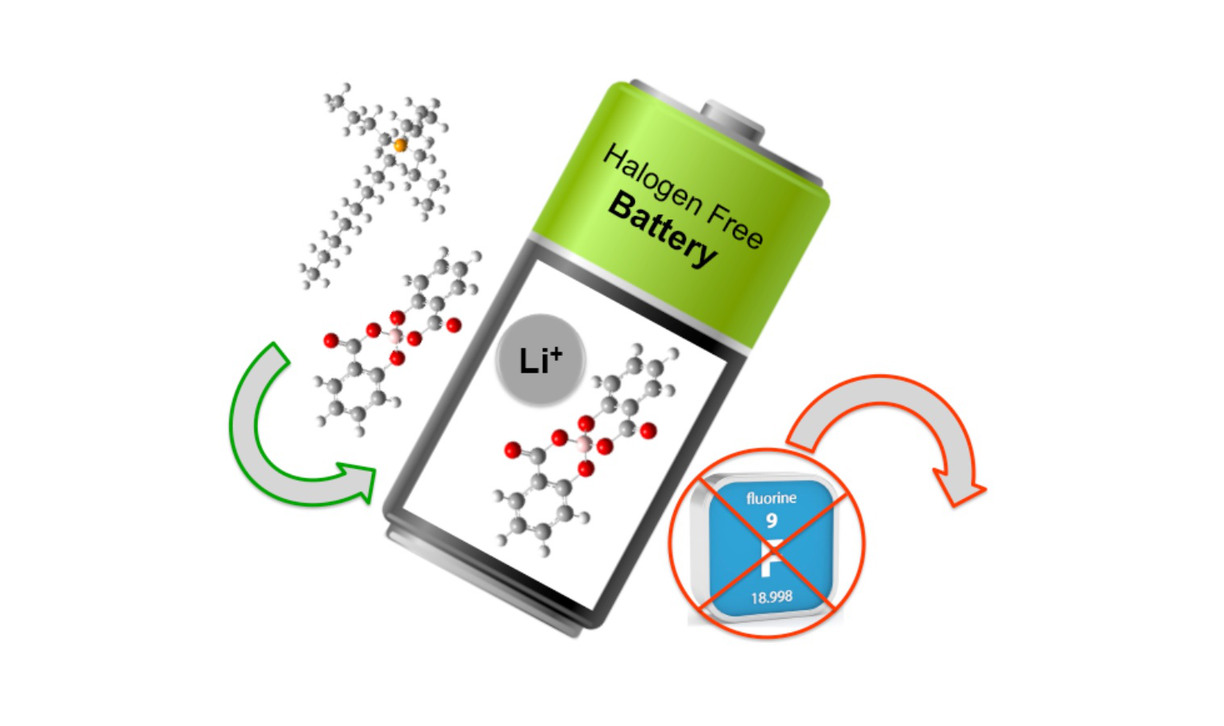
Enabling Safer Batteries via Fluorine-Free Electrolytes
One of the biggest challenges for next generation battery technology is the lack of appropriate electrolytes. Conventional organic solvent based electrolytes have a number of drawbacks such as toxicity, risk of leakage, high flammability, high vapour pressure, and low thermal and modest electrochemical stability.
The battery packs, made of hundreds to thousands of cells, contains flammable liquid electrolyte with a fluorinated lithium salt: LiPF6. Upon large heat generation or short-circuit the electrolyte might be ignited and burst into flames releasing toxic gases such as HF and POF3. There is an urge for replacing the flammable and fluorinated electrolytes of today with non-flammable and fluorine-free electrolytes in order to improve both safety and performance of our next generations of batteries.
Here we develop novel electrolytes based on fluorine-free alkali metal salts and ionic liquids. The alkali metal salts based on Li, Na and K as cations are dissolved in ionic liquids to create electrolytes. The overall design is based on fluorine-free anions having different flexible alkyl chains, enabling free rotators, many conformational degrees of freedom, and asymmetry. All electrolytes are extensively tested in various lab-cell concepts including assessing the stability of the electrolyte-electrode interfaces and interphases.
The electrolytes are characterized using multinuclear liquid Nuclear magnetic resonance spectroscopy (NMR), and solid-sate NMR, Pulse-Field Gradient (PFG) NMR, FTIR, MS, Raman spectroscopic techniques. Important physicochemical properties of newly synthesized electrolytes such as thermal behaviour (thermal stability, glass-transition, solid-solid transition (for plastic crystals) and decomposition temperatures) are measured using Thermogravimetric Analysis/Differential Scanning Calorimetry (TG/DSC), while density and viscosity are determined using densitometer and microviscometer, water content are determined using Karl Fisher titration. The electrochemical properties are characterized using potentiostat/galvanostat coupled with an Autolab Microcell HC for temperature-controlled experiments.
The project is financed by the Swedish Energy Agency.
Project leader: Associate Professor Faiz Ullah Shah
Project leader: Professor Patrik Johansson (Chalmers University of Technology)
Participant: Mukhtiar Ahmed (PhD student)
Contact
Faiz Ullah Shah
- Professor
- 0920-491291
- faiz.ullah@ltu.se
- Faiz Ullah Shah
Updated:
